How Safe is Generic Beta-Alanine? Key Takeaways from NPI’s Series
In a four-part series, the Natural Products Insider (NPI) sheds light on the ongoing battle within the FDA and the dietary supplements industry: efficacy in the enforcement of consumer protections. To date, Natural Alternatives International (NAI) is the only company that has adhered to the requirement of submitting a new dietary ingredient notification (NDIN) to the FDA for beta-alanine. The articles reference a 26-year-old U.S. law enacted to flag noncompliant novel ingredients for the purpose of initiating a premarket safety review. For over a year, however, the extensive efforts coordinated by Dan Fabricant, President and CEO of the Natural Products Association (NPA), and Kevin Bell, outside counsel to NPA, have yet to yield an FDA import alert against generic forms of beta-alanine made in China.
Investing in the Safety of Consumer Products
In a letter to Steven J. Tave, Director of the Food and Drug Administration, dated February 24, 2020, Bell wrote, “[NAI] spent hundreds of thousands of dollars to not only compile publicly available information about the ingredient’s identity, manufacturing process and safety, but also to conduct its own commercially confidential, preclinical studies. The agency did not object to NAI’s basis for concluding that CarnoSyn beta-alanine is reasonably expected to be safe, as manufactured, and under the conditions of use proposed in the notification.”
Compiling information for its beta-alanine NDI notification (NDIN) was a process that took NAI nearly a year and an investment of roughly $1 million for the process of compiling hundreds of pages of documents regarding testing, manufacturing methods, and safety. That figure more than doubles when including the human clinical trials conducted to support components of the submission.
Fundamental Concerns Over Consumer Protections
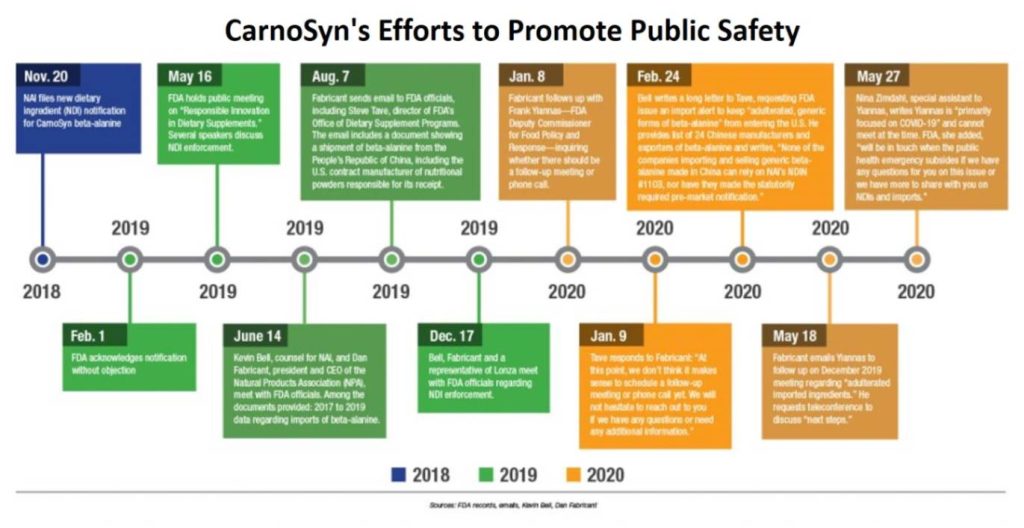
Twenty-five days after receiving confirmation that his company had satisfied the requirement in the law to establish a supplement containing an NDI, Mark LeDoux, Chairman and CEO of NAI, met with FDA officials, including Tave, “to discuss policing those products that were piggybacking on our successful NDI to import their beta-alanine without submitting safety or process data.” According to LeDoux, NAI’s proven safety at certain levels for its ingredient is not an invitation for other manufacturers to rely upon that evidence.
According to Fabricant, in an Oct. 4, 2019 email addressed to Frank Yiannas, FDA Deputy Commissioner for Food Policy and Response, “This creates a completely unbalanced playing field, effectively sending the message to U.S. companies that successfully submit an NDI that the agency is fine with someone claiming to have the exact same material as a company that submitted, without any evidence to show on that front.”
Commenting on the inequities, LeDoux explained, “There’s a fundamental concern here. Either the system is broken or it’s unenforceable. And if it’s unenforceable, then it needs to be fixed legislatively and/or administratively.” LeDoux further articulated that “it begs the question: Why would you file an NDI if the agency isn’t going to protect your efforts?”
On May 16, 2019, at a public meeting hosted by the FDA, “Responsible Innovation in Dietary Supplements”, Tave explicitly reminded the dietary supplements industry that “an effective NDI notification process represents FDA’s only opportunity to evaluate the safety of a new dietary ingredient before it becomes available to consumers.”
Bell followed up in February of 2020 in an eight-page letter to Tave, and again in May, requesting that the FDA enforce against “adulterated” beta-alanine being imported into the U.S. by 24 Chinese manufacturers. He provided their addresses, websites, kilograms of beta-alanine exported to the U.S. and total shipments between Feb. 1, 2019 and Jan. 31, 2020. Tave was also provided with the names of U.S. ingredient suppliers and contract manufacturers, as well as a list of companies selling finished brands that incorporate generic beta-alanine into their supplements. However, Tave maintains that there are no violations of law linked to the records that were shared.
Potential NDI Enforcement Against China-Manufactured Beta-Alanine
While the FDA has not committed to taking any action, it is still possible to see NDI enforcement against beta-alanine produced overseas. One such possibility is the issuing of an import bulletin. This would initiate the collection of beta-alanine samples for review and lab studies. If a violation of the law is suspected after such a review, an import alert could be triggered, identifying the products coming into the U.S. Ultimately, if the products are found to be under violation of the law, the U.S. supply of beta-alanine from China would be severed.
However, in response to a Freedom of Information Act (FOIA) submitted by Natural Products Insider, the FDA has not recently detained any products containing beta-alanine. Review of FDA inspection records, as well as Tave’s remarks, indicate that regulatory compliance inspections have not been conducted in recent years. Unfortunately, under the current global health crisis, the FDA has an abundant supply of “low” and “middle-hanging fruit”, as explained by Rick Collins, Partner at Collins, Gann, McCloskey & Barry, PLLC. “But that feeds into the perception by many in industry that FDA’s resources are not sufficient to go after other than the most egregious offenders,” said Collins.
Collins claims that it’s possible that the FDA is not yet aware of the risks posed by generic forms of beta-alanine manufactured in China—risks that could lead to a health crisis. He cited an example of the 1989 outbreak of eosinophilia-myalgia syndrome (EMS) found in users of L-tryptophan. This ingredient was an amino acid that was sold as a supplement and traced directly to a company using “recombinant technologies.” As previously cautioned by Bell, “Similar technologies deployed by Chinese firms making beta-alanine without going through the NDI process are rife with unnecessary risk.”
Click here to read the full four-part series of articles.